Left Atrial Appendage Exclusion. 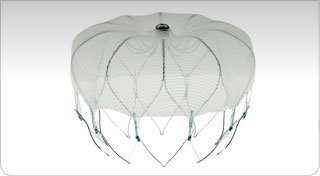
It is estimated that 2.2 million people in America and 4.5 million in the European Union have paroxysmal or persistent atrial fibrillation. Without anticoagulant therapy the risk of stroke in this population is about 5% per year in patients under 65 years and it increases to over 8% per year in patients over 75.
In patients with atrial fibrillation, the left atrial appendage (LAA) provides relatively stagnant rheologic environment favouring the development of thrombi, which may embolize to the brain or other vital organs.
Effected patients are routinely treated with anticoagulants (Coumadin® or Marcumar®), which are characterized with a narrow therapeutic window requiring frequent monitoring and dose adjustment. The risks of a bleeding events are significant.
Alternate treatments involve antiarrhythmic interventional treatment targeted to reverse reentry pathways supporting paroxysmal or persistent atrial fibrilation – OR – placement of a device to close the aperture of the LAA.
Over many years, TheraGenesis has contributed to the clinical development and regulatory approval of this closure device.