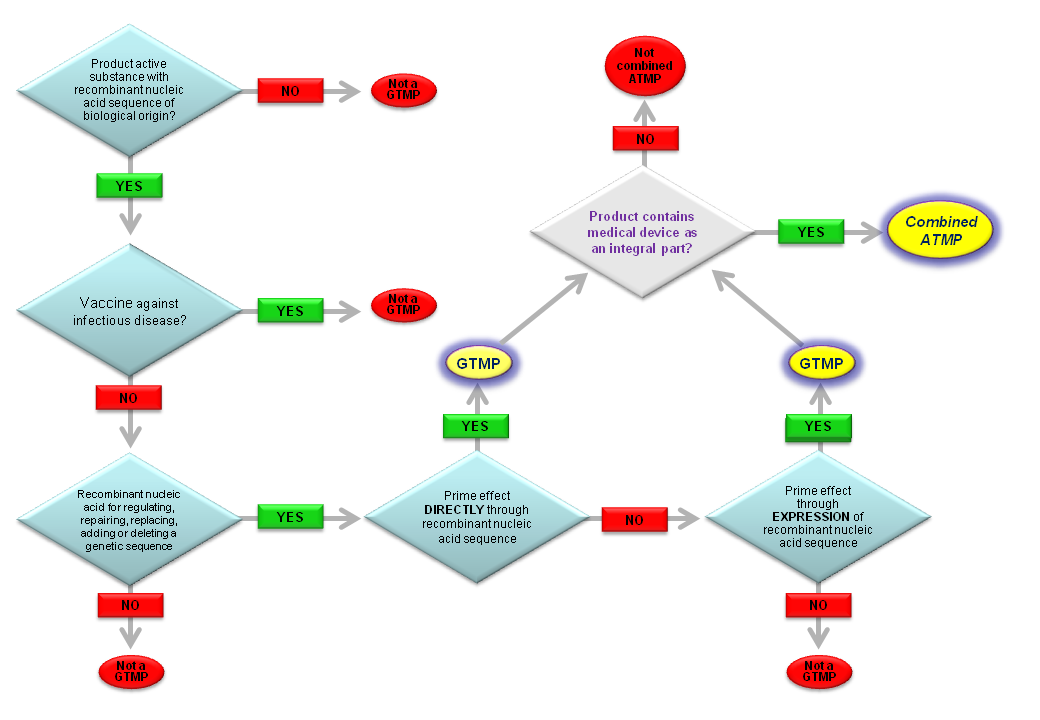
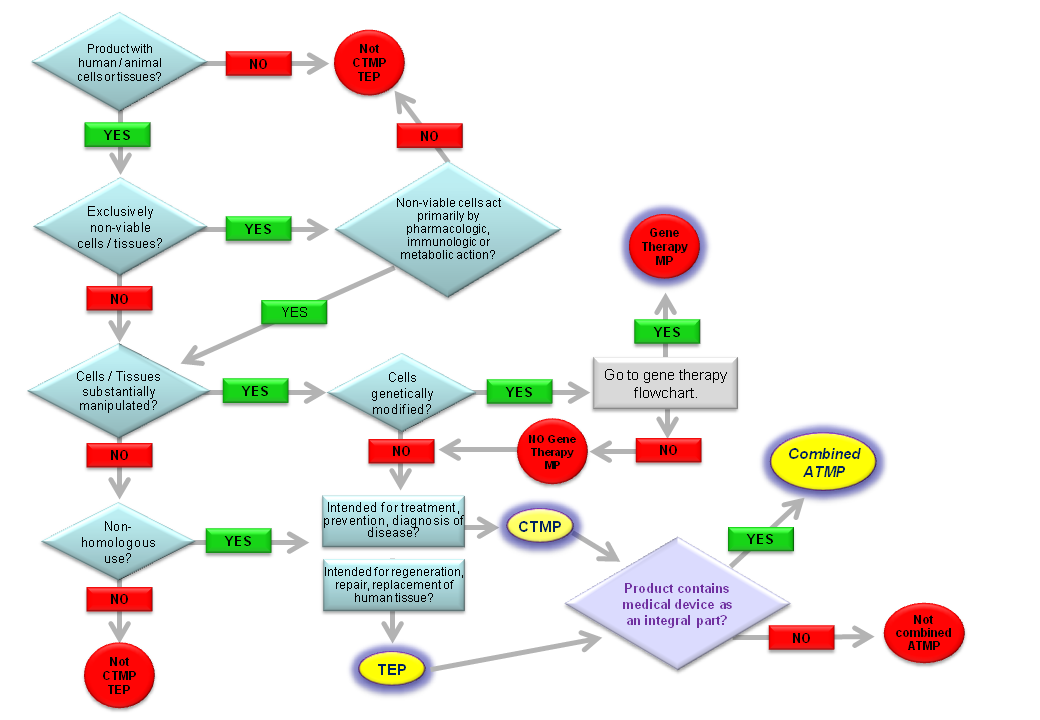
1. (b) ‘Tissue engineered product’ means a product that:
- contains or consists of engineered cells or tissues, and
- is presented as having properties for, or is used in or administered to human beings with a view to regenerating, repairing or replacing a human tissue.
A tissue engineered product may contain cells or tissues of human or animal origin, or both. The cells or tissues may be viable or nonviable. It may also contain additional substances, such as cellular products, biomolecules, biomaterials, chemical substances, scaffolds or matrices.
Products containing or consisting exclusively of nonviable human or animal cells and/or tissues, which do not contain any viable cells or tissues and which do not act principally by pharmacological, immunological or metabolic action, shall be excluded from this definition.
(c) Cells or tissues shall be considered ‘engineered’ if they fulfill at least one of the following conditions:
- the cells or tissues have been subject to substantial manipulation, so that biological characteristics, physiological functions or structural properties relevant for the intended regeneration, repair or replacement are achieved. The manipulations listed in Annex I, in particular, shall not be considered as substantial manipulations,
- the cells or tissues are not intended to be used for the same essential function or functions in the recipient as in the donor.
ANNEX I:
Manipulations referred to in the first indent of Article 2(1)(c)
cutting, grinding, shaping, centrifugation, soaking in antibiotic or antimicrobial solutions, sterilization, irradiation, cell separation, concentration or purification, filtering, lyophilization, freezing, cryopreservation, vitrification.