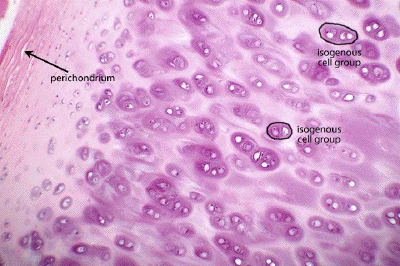
 TheraGenesis collaborated with a biotech manufacturer to obtain european regulatory approval of an autologous chondrocyte implantation product. The product is the first and only FDA-approved cell therapy product used to repair articular cartilage injuries in the knee of adults who have not responded to a prior arthroscopic or other surgical repair procedure. The process involves the removal of healthy chondrocytes from the lesioned joint, cell-culture on a scafold and reintroduction over the lesion. The implanted cells can form new hyaline-like cartilage, with properties similar to normal cartilage which may reduce pain and improve knee function.
TheraGenesis collaborated with a biotech manufacturer to obtain european regulatory approval of an autologous chondrocyte implantation product. The product is the first and only FDA-approved cell therapy product used to repair articular cartilage injuries in the knee of adults who have not responded to a prior arthroscopic or other surgical repair procedure. The process involves the removal of healthy chondrocytes from the lesioned joint, cell-culture on a scafold and reintroduction over the lesion. The implanted cells can form new hyaline-like cartilage, with properties similar to normal cartilage which may reduce pain and improve knee function.